From Our CEO
Read a letter from CLSI's CEO, Dr. Barb Jones, about the recent FDA final rule on the regulation of Laboratory Developed Tests (LDTs).

CLSI QSRLDT
Quality System Regulations for Laboratory-Developed Tests: A Practical Guide for the Laboratory, 2nd Edition
This practical guide, compiled with the help of experts from the in vitro diagnostics industry, is intended for the laboratory that is creating laboratory developed tests that may be subject to the US Food and Drug Administration (FDA) regulations, specifically the Quality System Regulation (QSReg), 21 CFR Part
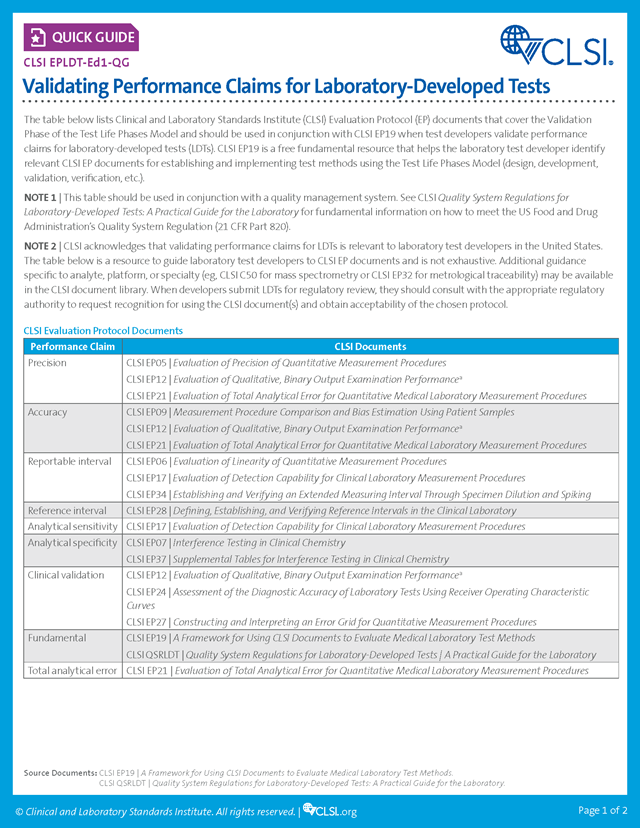
CLSI EPLDT Quick Guide
Validating Performance Claims for Laboratory-Developed Tests, 1st Edition
This quick guide lists CLSI Evaluation Protocol documents that cover the Validation Phase of the Test Life Phases Model.
Be Prepared for FDA Oversight
NEW! CLSI Perspective
The FDA Ruling on LDTs:
Taking Control of What's in Your Control
Since the final FDA ruling on Laboratory Developed Tests was published on 06 May 2024, there has been a great deal of discussion around the interpretation and implications of the new oversight. The final ruling declares the FDA’s authority to regulate laboratory developed tests (LDTs) as medical devices under the Food, Drug, and Cosmetic Act (FDCA) and phases out the discretionary enforcement that has been in place since the law was first enacted in 1976.
For 60 years CLSI has served as a trusted resource for laboratories, and we're here for them now – with the guidance, tools, and resources to ensure your lab is prepared. Download the report below for more information about how to navigate these new requirements.
On-Demand & Upcoming Webinars | Register today for these upcoming webinars

CLSI M100 On-Demand 2025 Update Webinar
Implement CLSI M100 With Confidence
Join CLSI on Wednesday, February 26 at 1:00 ET for a webinar on the latest in Antimicrobial Susceptibility Testing (AST) with the release of CLSI M100 Ed35. Receive the latest guidance for implementing new breakpoints and standards into your laboratory practices, including discussion of the major changes in this edition

CLSI On-Demand Regulatory Webinar
Streamline the FDA Approval Journey: A Panel Discussion with the FDA, CLSI, and Abbott Laboratories
Navigating the FDA device approval and clearance process can be daunting. However, the appropriate use of consensus standards can greatly reduce the burden for the conformity assessment elements of medical device submissions. By using declarations of conformity (DOC), particularly with FDA-recognized standards, device developers and manufacturers can streamline submission preparation. The session will provide direction on how stakeholders can – and should – contribute to the development of conse

CLSI On-Demand EP 2024 Webinar
What's New in Method Evaluation?
Participate in this CLSI webinar to gain knowledge about the latest method evaluation standards, get a sneak peek of EP47, and engage in a Q&A session addressing important topics in the industry.

CLSI On-Demand LDT Webinar 1
LDT Foundations: An Overview of FDA's Final Rule on LDTs
Join CLSI for this webinar series dedicated to the stages of FDA's Final Rule on Laboratory Developed Tests (LDTs). Register for this first webinar and receive access to the entire series, which will provide practical guidance, resources, and vital information for laboratories. Once you are registered for the series, you will receive notifications about new webinars that are released, all on-demand recordings, and webinar slides. CLSI will update this series frequently with new webinar dates, to

CLSI On-Demand LDT Webinar 2
LDT Foundations: Standards to Prepare for Stage 1 Requirements

CLSI On-Demand LDT Webinar 3
LDT Foundations Webinar Series: Navigating Design Controls for LDTs
Join CLSI for a webinar series dedicated to the stages of FDA's Final Rule on Laboratory Developed Tests (LDTs). The third webinar is a discussion around navigating design controls for FDA's Final Rule on LDTs, including resources for your laboratory to implement into practice. The webinar will include guidance around design controls, a highlight of useful resources, and a Q&A discussion.
LDT Related Guidelines | Find all CLSI resources related to LDT
|
Product Code
|
Short Title
|
Publication Date
|
FDA Recognition Date
|
|---|---|---|---|
|
EP05-A3
|
Evaluation of Precision
|
2014-10-01
|
2015-08-14
|
|
EP06-Ed2
|
Evaluation of Linearity
|
2020-11-24
|
2021-06-07
|
|
EP06-Ed2-EG
|
Developer Validation of Linearity Establishment Guide
|
2024-09-05
|
2025-06-26
|
|
EP07-Ed3
|
Interference Testing
|
2018-04-30
|
2018-09-17
|
|
EP09-Ed3c
|
Measurement Procedure Comparison
|
2018-06-20
|
2020-07-06
|
|
EP12-Ed3
|
Qualitative Test Performance
|
2023-03-07
|
2023-05-29
|
|
EP14-Ed4
|
Commutability of Processed Samples
|
2022-07-14
|
|
|
EP17-A2
|
Evaluation of Detection Capability
|
2012-06-18
|
2013-01-15
|
|
EP18-A2
|
Risk Management Techniques
|
2009-11-30
|
2010-10-04
|
|
EP18-Ed2-EP23-Ed2-WS
|
Laboratory Quality Control Based on Risk Management; Worksheet Template
|
2024-06-28
|
|
|
EP19-Ed3
|
Using CLSI EP Documents
|
2022-10-10
|
2024-12-23
|
|
EP21-Ed2
|
Total Analytical Error
|
2016-07-01
|
2016-12-23
|
|
EP23-Ed2
|
Laboratory QC Based on Risk Management
|
2023-08-15
|
|
|
EP23-Ed2-QG
|
EP23 Quick Reference Guide
|
2024-06-27
|
|
|
EP23-Ed2-WB
|
Laboratory Quality Control Based on Risk Management; Workbook
|
2024-06-26
|
|
|
EP24-A2
|
Diagnostic Accuracy Using ROC Curves
|
2011-11-30
|
2013-08-06
|
|
EP25-Ed2
|
Reagent Stability
|
2023-04-26
|
2023-12-18
|
|
EP27-Ed2
|
Constructing Error Grids
|
2022-06-14
|
2022-12-19
|
|
EP28-A3c
|
Reference Intervals
|
2010-10-19
|
2014-01-30
|
|
EP30-Ed2
|
Commutable Reference Material
|
2024-08-01
|
|
|
EP34-Ed1
|
Dilution and Spiking for Extended Measuring Interval
|
2018-08-13
|
2019-07-15
|
|
EP35-Ed1
|
Equivalency of Specimen Types
|
2019-12-19
|
2020-07-06
|
|
EP37-Ed2
|
Interference Testing Tables
|
2024-10-15
|
|
|
EP39-Ed1
|
Surrogate Samples
|
2021-11-11
|
2021-12-20
|
|
EPLDT-QG-Ed1
|
Validating Performance Claims for Laboratory-Developed Tests
|
2024-07-01
|
|
|
MN v.2
|
Unchanged Test Methods Workbook
|
2024-07-17
|
|
|
BPI Toolkit
|
BPI Toolkit
|
2023-06-08
|
|
|
M47-Ed2
|
Blood Cultures
|
2022-04-22
|
2024-12-23
|
|
M58-Ed1
|
MALDI-TOF MS
|
2017-04-26
|
2017-08-21
|
|
MM01-Ed4
|
Molecular Genetics/Specimen ID
|
2023-06-26
|
|
|
MM06-A2
|
Quantitative Molecular InfDis
|
2010-11-30
|
2013-01-15
|
|
MM09-Ed3
|
DNA Sequencing
|
2023-04-07
|
|
|
MM17-Ed2
|
Molecular Multiplex Validation
|
2018-05-31
|
2019-07-15
|
|
MM21-Ed1
|
Genetic/Oncology Microarrays
|
2015-08-28
|
2016-06-27
|
|
MM22-Ed2
|
Infectious Disease Microarrays
|
2024-01-24
|
|
|
MM24-Ed1
|
Genotyping of Infectious Organisms
|
2021-09-30
|
2024-05-29
|
|
QMS01-Ed5
|
Quality Management System
|
2019-06-19
|
2019-12-23
|
|
QMS02-Ed7
|
Laboratory Documents
|
2024-03-12
|
|
|
QMS05-Ed3
|
Referral Laboratories
|
2020-06-02
|
|
|
QMS06-A3
|
Continual Improvement
|
2011-06-30
|
2014-01-30
|
|
QMS11-Ed2
|
Nonconforming Events
|
2015-08-25
|
|
|
QMS14-Ed2
|
Leadership/Management Roles and Responsibilities
|
2024-10-18
|
|
|
QMS18-Ed2
|
Process Management
|
2023-05-31
|
|
|
QMS21-Ed1
|
Purchasing and Inventory
|
2016-11-01
|
|
|
QMS22-Ed1
|
Laboratory Information Management
|
2018-08-30
|
|
|
QMS25-Ed1
|
Quality Manual
|
2017-01-23
|
|
|
QMS26-Ed1
|
Managing Laboratory Records
|
2021-04-14
|
|
|
QSRLDT-Ed2
|
Laboratory-Developed Tests
|
2024-09-16
|
|
|
MM23-Ed2
|
Molecular Diagnostic Methods for Solid Tumors
|
2025-02-11
|
|
|
EP35-Ed2
|
Equivalency of Specimen Types
|
2025-03-03
|
|
|
EP21-Ed3
|
Total Analytical Error
|
2025-06-16
|
|
|
EP46-Ed1
|
Determining TAE Goals
|
2025-06-16
|
|
|
EP21-Ed3-QG
|
Total Error Quick Guide
|
2025-06-16
|
|
|
EP46-Ed1-QG
|
Determining Error Goals and Limits Quick Guide
|
2025-06-16
|
|
|
MM14-Ed3
|
Molecular PT
|
2025-07-17
|
|
|
QMS06-Ed4
|
Continual Improvement
|
2025-07-28
|
|
|
EP14-Ed4-G
|
Evaluation of Commutability Guide
|
2025-10-14
|
|
|
BIT-Ed2
|
BPI Toolkit
|
2025-10-14
|
|
Manage Regulatory Requirements With the Newly Updated Method Navigator Tool
As a member of the IVD community, your test methods are subject to myriad regulatory requirements. It can be difficult to stay up-to-date or know which guidance to follow. CLSI’s newly updated Method Navigator, a comprehensive resource that maps our specific guidance to meet regulatory requirements, was built to help those who develop both IVDs and LDTs. Streamlined navigation to newly published revisions of CLSI documents including EP19, EP12, EP25, QMS17, and QMS18 is one key update that we encourage you to take advantage of!
Prepare Your Lab With These CLSI Resources

View our full library here

Become a member to have access to these resources and more!
Add LDT and EP to Your CLSI Areas of Interest

Crosswalk of Documents Referenced Within FDA Accreditation Checklists

NEW! Review our LDT Fact Sheet

View CLSI LDT Response Documents
FDA LDT Resources

FDA LDT Final Rule

Laboratory Developed Tests: FDA FAQs