Regulation of Laboratory Tests Is About to Change
3/24/2020
If Congressional representatives Diana DeGette (D-CO), Larry Bucshon, MD (R-IN), and Senators Michel Bennet (D-CO) and Orrin Hatch (R-UT) get their way, in vitro clinical tests (IVCTs) and laboratory developed tests (LDTs) will soon all be regulated in a whole new way.
The bipartisan bill, Verifying Accurate Leading-edge IVCT Development Act (VALID), was introduced to Congress on March 5, 2020. This legislative effort, if enacted, will overhaul the regulatory oversight of in vitro diagnostics (IVDs) in the United States and create a single system for regulation of LDTs and manufacturers’ IVCTs.1 The VALID Act creates a risk-based framework for regulation, with high-risk tests, like novel assays, required to go through FDA premarket review, while lower-risk tests could go to market after passing through technological certification. Unlike previously circulated discussion drafts, the introduced bills include specific language designed to address public health emergencies, including COVID-19.
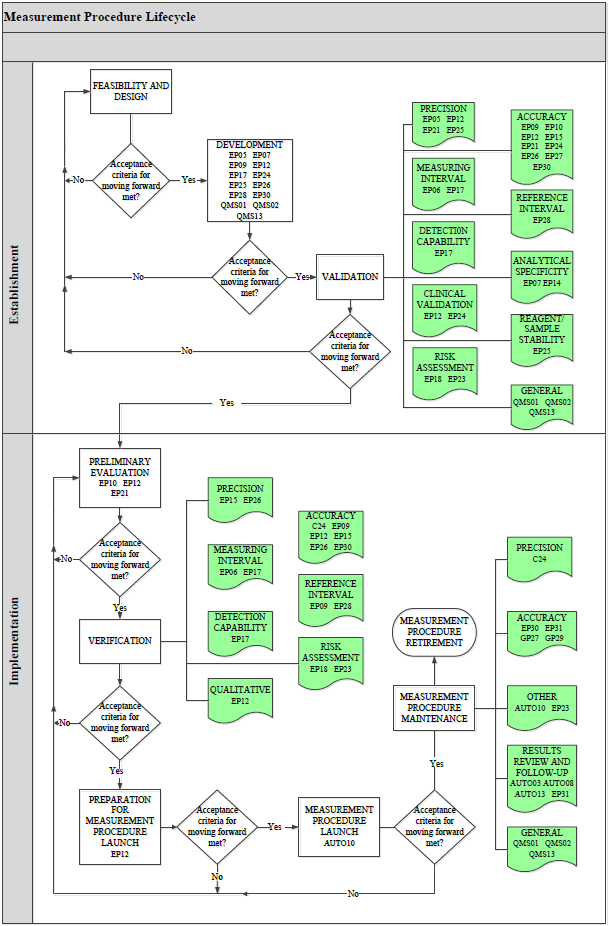
All IVDs, including IVCTs and LDTs, can be described using a measurement procedure lifecycle model. Laboratory measurement procedures begin with a concept, and proceed through development, validation, verification, launch, and, finally, retirement. At each stage of its lifecycle, the success of a measurement procedure hinges upon a series of stage-dependent requirements such as scientific and process feasibility, quality system requirements, and business constraints (eg, time, resources).
Clinical and Laboratory Standards Institute offers a free document EP19—A Framework for Using CLSI Documents to Evaluate Clinical Laboratory Measurement Procedures that describes this lifecycle concept and serves as a reference guide for the use of CLSI documents for establishing and implementing IVDs. EP19 also explains helpful statistical concepts that are used in CLSI documents, including how they may be helpful in measurement procedure establishment and implementation. EP19 illustrates the usefulness of CLSI documents and provides helpful information for noncommercial laboratories that develop their own measurement procedures.
EP19 can be downloaded for free here. Additional method evaluation documents from CLSI are available for purchase on our website.
1. The VALID Act, Aiming to Reform the Regulation of Diagnostic Products, Is Finally Introduced in Congress.The National Law Review. Accessed March 23, 2020.
