CDC Issues Health Alert for Extensively Drug Resistant Shigella
3/2/2023
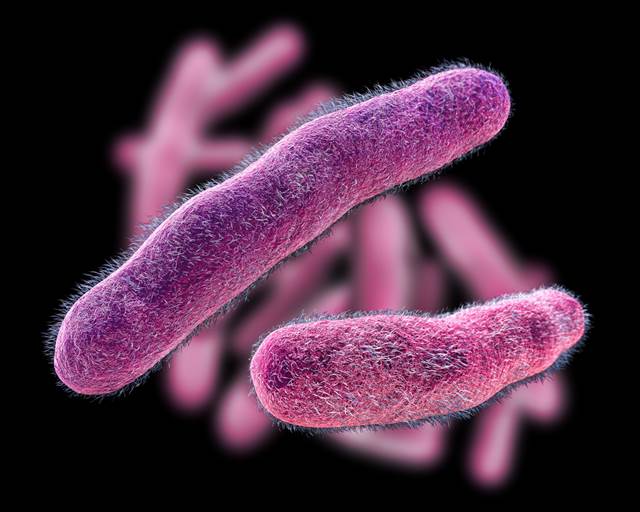
The Centers for Disease Control and Prevention (CDC) has issued a health alert after observing a sharp increase in the number of cases of extensively drug-resistant (XDR) Shigella–a bacteria that causes shigellosis, a major form of inflammatory diarrhea. According to the CDC, the proportion of Shigella infections in the US that are resistant to all known antibiotic treatments rose from zero in 2015 to 0.4% in 2019, before leaping to 5% last year.
An estimated 77,000 antibiotic resistant Shigella infections occur in the United States each year. Shigella is considered XDR when it is not susceptible to any of the recommended first-line or alternative antibiotics, including azithromycin, ciprofloxacin, ceftriaxone, trimethoprim-sulfamethoxazole, and ampicillin. Given these potentially serious public health concerns, the CDC is asking healthcare professionals to be vigilant about suspecting and reporting cases of XDR Shigella infection to their local or state health department. They are also urging patients and communities at increased risk to educate themselves about prevention and transmission of the infection.
The World Health Organization (WHO) has identified drug-resistant pathogens as one of the top 10 global public health threats facing humanity. The emergence of these pathogens is largely driven by the misuse and overuse of antibiotics in people and livestock. It is believed that AMR was the direct cause of at least 1.27 million deaths in 2019. According to WHO, the number is expected to increase to 10 million by 2050 if urgent action is not taken to address the problem.
CLSI will publish the 33rd Edition of M100—Performance Standards for Antimicrobial Susceptibility Testing this month. M100 helps laboratorians provide test results that health care providers use to select appropriate antimicrobials to treat their patients. The document is intended for use by microbiology labs, antimicrobial stewardship teams, infectious disease experts, and other relevant stakeholders. The tables presented in M100 represent the most current information for drug selection, interpretation, and quality control.
This year, a new table on antimicrobial agents for Salmonella and Shigella has been added to M100.
Interested in getting involved with CLSI's Susceptibility Testing Subcommittees? View more resources from them here.
