AST News Update June 2023: Aminoglycoside Case Study
6/21/2023
Graeme N Forrest, MBBS, Professor of Medicine, Division of Infectious Diseases, Rush University, Chicago, Illinois, USA
A 45-year-old patient with acute myeloid leukemia (AML) received induction chemotherapy 2 weeks ago and currently has an absolute neutrophil count of <100 cells/μL. The patient had been on levofloxacin prophylaxis for his neutropenia, but developed a fever a week ago, at which point meropenem was started. All cultures and imaging were negative.
The patient developed hypotension and was transferred to the intensive care unit, where he was intubated and placed on vasopressors. A chest x-ray showed dense right lower lobe pneumonia. Vancomycin and amikacin were added to the meropenem therapy a day later.
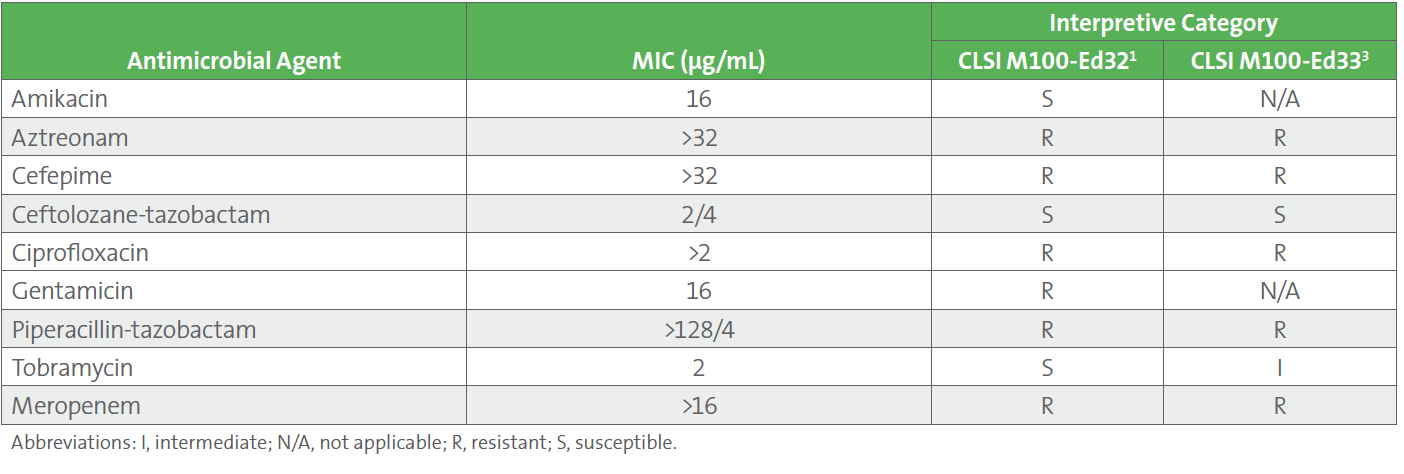
Blood and sputum cultures grew gram-negative rods, identified as Pseudomonas aeruginosa by rapid diagnostic testing. The patient’s condition continued to deteriorate and repeat blood cultures one day later grew P. aeruginosa. Unfortunately, the patient expired on day 3 of hospitalization. Final cultures grew carbapenem-resistant P. aeruginosa, susceptible to amikacin and tobramycin, but resistant to gentamicin by applying CLSI M100-Ed32 breakpoints (see Table 1).1
Important Takeaways
This case reflects 2 important points:
1. By applying the updated breakpoints published in CLSI M100-Ed33, tobramycin would have been the only aminoglycoside predicted to achieve bacterial stasis for systemic infections due to P. aeruginosa.2 It was inappropriate to use amikacin, since amikacin is effective against P. aeruginosa only in treatment of urinary tract infections.
2. When gram-negative bacteremia occurs as a breakthrough infection (ie, in a patient who is already receiving gram-negative-directed therapy such as this patient on levofloxacin), addition of an aminoglycoside to meropenem is not an appropriate therapeutic approach. Addition of an aminoglycoside would not add a significant amount of efficacy to the meropenem. Rather, the clinician should have treated with a different beta-lactam drug such as piperacillin-tazobactam or cefepime, or perhaps one of the newer beta-lactam inhibitor combinations (eg, ceftolozane-tazobactam or ceftazidime-avibactam).3
Changes to the aminoglycoside breakpoints require careful conversation with antimicrobial stewardship programs and possible revisiting of current clinical guidelines (such as those for the intensive care unit [ICU] where aminoglycosides are cornerstone antimicrobials for the management of gram-negative infections).
Table 1. AST Profile of the Pseudomonas aeruginosa Isolate
References
1 CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 32nd Edition. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
2 CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 33rd Edition. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2023.
3 Tamma P, Aitken S, Bonomo R, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021:72(7):e169-e183. Doi:10.1093/cid/ciaa1478.
