Volunteer
Volunteer to help shape laboratory standards.
Your expertise is in demand. Grab a seat at the table with your colleagues in the health care community and help develop the next generation of medical laboratory testing standards.


New to Volunteering at CLSI?
Our step-by-step guide to getting involved.

Already a CLSI Volunteer?
Thanks for your contributions. Access your committee work now.
Why Volunteer?

Share Your Expertise
Take part in developing the best practice standards that affect the work you do every day.

Enhance Your Career
Grow your professional experience and résumé.
Gain Recognition
Get noticed by CLSI, your organization, your colleagues, and your community.
Network & Connect
Meet people who will be great contacts forever.
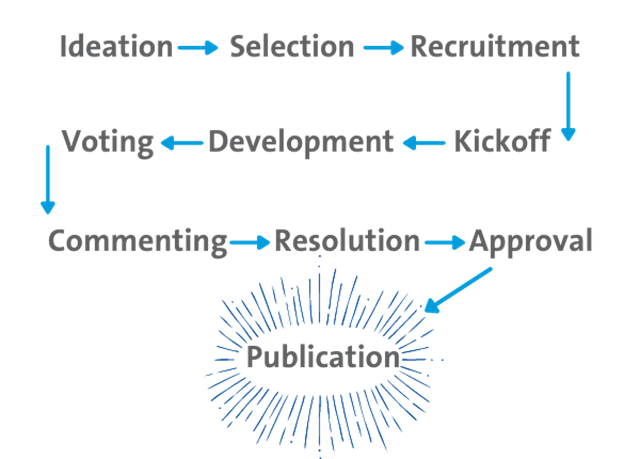
How CLSI Standards Are Developed
Our volunteers bring to our standards development process diverse skills, deep experience, and vast knowledge.
"If you're a person who cares about laboratory medicine, about the quality of the results that get to the patient...you really need to volunteer."
Andrew Quintenz
Board of Directors Member, CLSI
Global Scientific & Professional Affairs Manager, Bio-Rad Laboratories, Inc.
Current Opportunities
Don’t see an opportunity in your area of expertise? Complete your volunteer profile to get updates about new opportunities when they’re posted.

M68
Validation of Commercial Antimicrobial Susceptibility Testing Breakpoints, 1st Edition
Apply on or before 5 July 2023.
This group is looking for volunteers with:
-
Expertise in AST
-
Experience in developing AST breakpoints
-
Familiarity with and practical use of CLSI and FDA breakpoints
-
Knowledge of Clinical Laboratory Improvement Amendments (CLIA) requirements for test validation

This group is looking for volunteers with:
-
Expertise in molecular oncology
-
Experience with liquid biopsy molecular testing methods
-
Proficiency in liquid biopsy test development
-
Knowledge of regulatory compliance and quality management in molecular testing
How to Volunteer

Create an Online Account or Log in
It’s easy, free, and takes only a few minutes. Already have an account? You can login here too.

Complete Your Volunteer Profile
Once you complete your profile, you’ll receive updates about opportunities in your area of interest.

Apply for Current Opportunities
CLSI sends new volunteer opportunities every month via e-mail. You can also check back here or in your CLSI Exchange account to view opportunities.

Get Notified About Your Application
If selected, you’ll be notified about your committee position and start date via e-mail. You can learn more about the volunteer process at our Standards Development Process page.
Qualifications
Our volunteers come from diverse professional backgrounds and include health care, government, and industry professionals with the common goal of improving the quality of medical laboratory testing.
- Familiarity with CLSI’s standards.
- Experience in that area of laboratory science (varies by opportunity).
- Ability to work well in a group setting during the time frames listed on the opportunity.
- You don’t need an advanced degree to participate.
Payment of an annual administrative fee is required for participation on CLSI committees. If you or your organization is a member of CLSI, the annual fee is included in your membership dues and no additional payment is required. More information on CLSI membership can be found here.
What to Expect
On average, it takes 18-24 months to complete a CLSI document. As a committee volunteer, you are asked to commit to participating for the duration of the project.
Volunteers meet regularly—in person and via conference call—throughout the development process to review the draft document, discuss and resolve issues related to the document's content, and to make sure the committee is on schedule with respect to the document development timeline.
Groups & Committees
Working Groups
CLSI's working groups focus on tasks that support the work of a subcommittee. A working group's assignment is usually limited in scope. They can include writing a document, a section of a document, or conducting a technical study.
Subcommittees
Subcommittees are responsible for documents in a topic area. They write draft documents, evaluate and respond to comments on content throughout the document development process, conduct scheduled document reviews, and may be responsible for continual revision of certain documents.
Document Development Committees
Comprised of a balanced representation from government, industry, and the health care professions, a CLSI document development committee is a group of technical experts who work together to develop a CLSI document.
Expert Panels
Expert panels are responsible for identifying and proposing projects and advising the Consensus Council on their suitability. Their focus is on reviewing, commenting on, and voting on documents within their area of expertise.
Consensus Council
The Consensus Council sets priorities for and manages CLSI standards development and identifies continual improvement opportunities for standards development-related processes. The Consensus Council also votes on Final Draft documents to confirm adherence to process requirements.
Board of Directors
The CLSI Board of Directors fulfills CLSI's mission by supervising, controlling, and directing CLSI's affairs; assuring the integrity of the voluntary consensus process.

